Related: Lab Management MOC, Hardwater into dishwasher and milliq machine ruining machines
Summary
Milli-Q water is a water purification system for labs which include high-flow and ultrapure water systems. It removes contaminants/impurities and distributes water free from particles, ions, and other organic compounds.
Side note: do not drink this, or use the Ultrapure water for equipments!
Purity via reverse osmosis, de-ionization, and UV irradiation
MilliQ water is purified to Type I standard = very low impurities. It goes through a multi-step process (see heading).
Type 1 = Ultrapure Water for highly sensitive applications
Type 2 = General laboratory use
Q-pod for Ultrapure water (Type 1) + UV Lamp
E-pod for General Lab use and Instrument Feed (Type 2) + UV Lamp
Reverse osmosis (RO)
Separation of water molecules from other substances using a semi-permeable membrane. This even works for dissolved/suspended compounds (like chemicals or other bio substances like bacteria). The membrane is too small for the other compounds to move through, but water can pass through it easily.
Osmosis is the diffusion of water down the concentration gradients/membranes, so reverse osmosis is just separating the water from other ions.
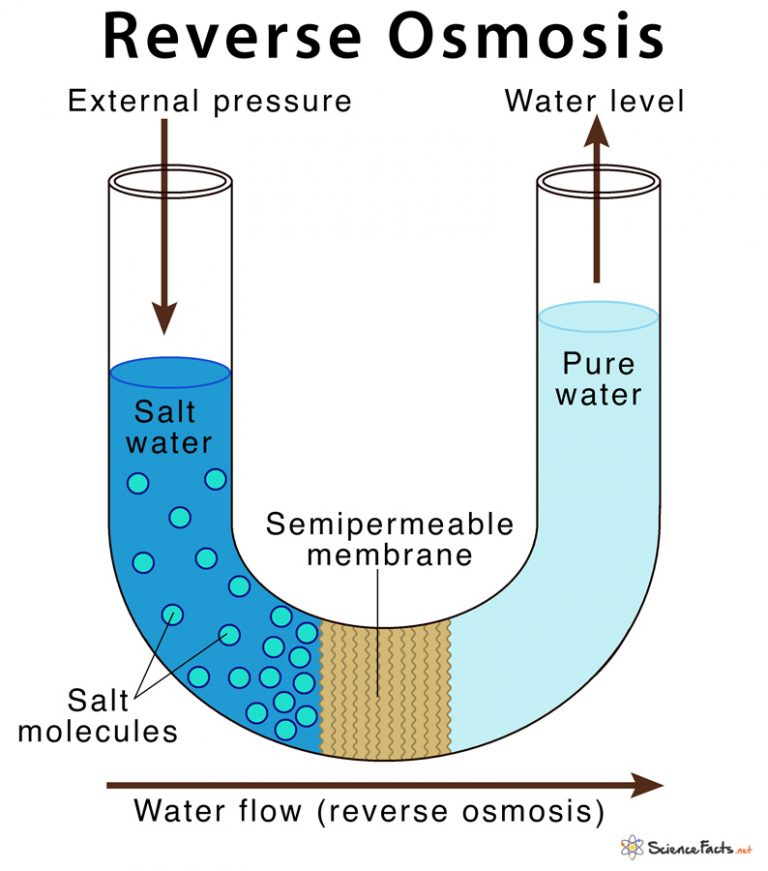
picture source - Science Facts
Deionization
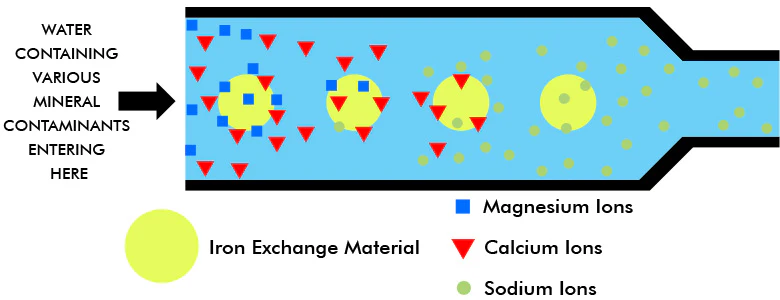
This is the process to remove all dissolved salts. For deionization to work, the water must go through two ion exchange components.
Step 1: Releasing H+ (hydrogen) ions
The metallic ions in water stick themselves to the “exchange material,” and then the exchange material releases a proportionate amount of hydrogen ions. This balances the overall electrical charge.
Examples: A sodium ion (Na+) displaces one hydrogen ion (H+); Calcium ion (Ca+) displaces 2, Ferric ion (Fe+++) displaces 2, etc.
At this point, the water is partially treated because the positive metallic ions are removed. However, with a bunch of new H+ ions, it’s also become acidic. To fix it, we go to step 2.
Step 2: releasing OH- (hydroxyl) ions
The water treated in step 1 goes to another unit with anion exchange material. This exchange material usually has replaceable hydroxyl anions and fixed irreplaceable cations.
Now the negative ions (anions) are absorbed into the anion exchange material, and OH- ions are released in its placed.
What flows out of this now is ion-free water. The H+ ions and the OH- ions form together to make water (H2O) that’s not different from the way they were produced (besides now being contaminant and ion free, whereas the water at the beginning was neither!)
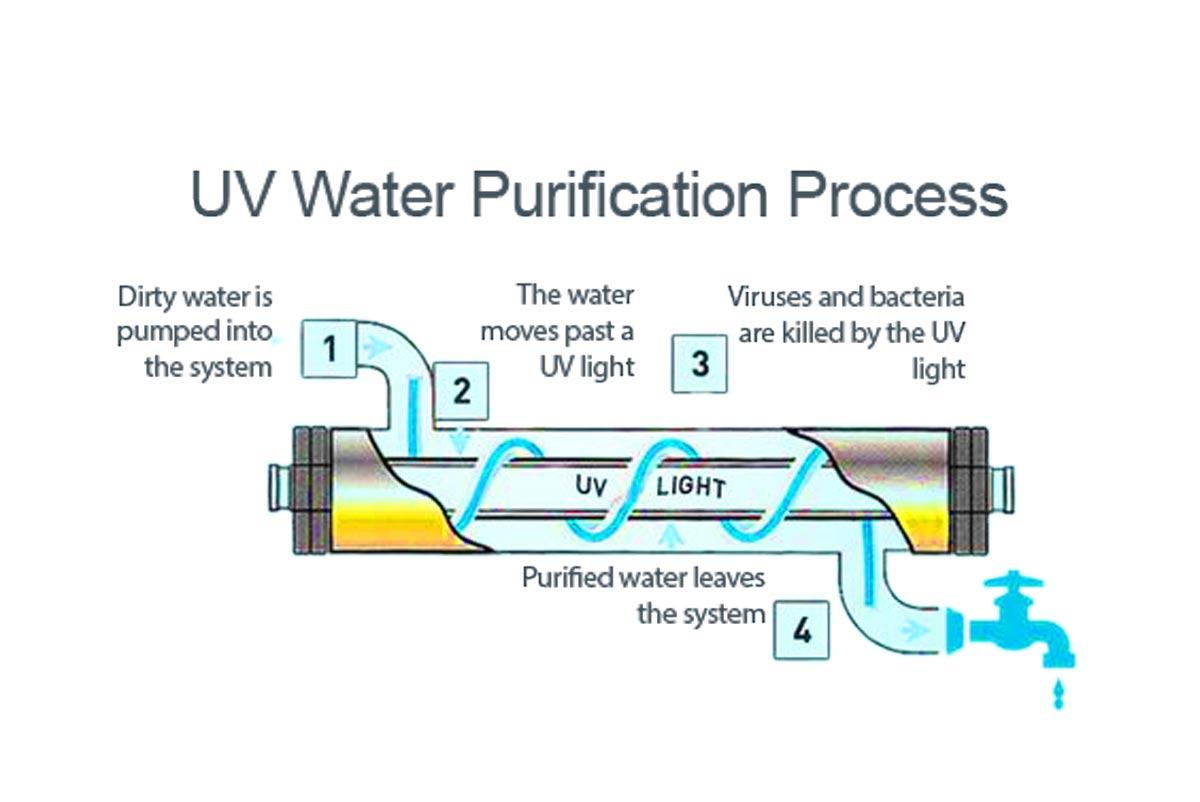
UV Irradiation
The water from step 2 is pumped through a tube or new chamber, past a UV light. The UV light kills UV-sensitive microorganisms (like Viruses and bacteria), and exits through the dispenser, purified.